An Auspicious Day
Robert Koch presented his investigations into the microbiological cause of tuberculosis on 24th March 1882, at a meeting held at the Berlin Physiological Society. (Centers for Disease Control and Prevention 1982) That auspicious day has been commemorated by the World Health Organization (WHO) as World TB Day, an occasion on which healthcare providers and the general public take stock of the impact which Mycobacterium tuberculosis has on human health. (World Health Organization 2023)
Global Burden of Tuberculosis
The WHO reported a precipitous fall in the number of tuberculosis notifications worldwide in 2020, because of the COVID-19 pandemic. (World Health Organization 2022b) In 2019, 7.1 million cases of newly diagnosed tuberculosis were reported to the WHO and this declined to 5.8 million cases in 2020. The number of newly reported tuberculosis cases represents a fraction (57.4%) of the estimated global burden of 10.1 million persons who fell ill with tuberculosis in 2020. Principal reasons for the reduction in tuberculosis case reporting during 2020 were programmatic shifts which diverted attention away from routine clinical services towards containment and management of the COVID-19 pandemic. In addition, restricted access to healthcare facilities because of lockdown policies, coupled with public avoidance of health care services because of the perceived risk of contracting SARS-CoV-2 infection contributed to lower case detection rates. Global targets to control tuberculosis were derailed because of COVID-19 and may take years of intensified activity and increased investment in terms of funding to recover.
Eleven percent of all tuberculosis cases globally occur in children under 15 years of age. Children under 5 years are at particular risk of becoming sick with tuberculosis because of their relatively immature immune systems. Other risk factors for progression to disease after infection with M. tuberculosis include malnutrition and co-infection with human immunodeficiency virus (HIV). The WHO target for tuberculosis treatment coverage was for 3.5 million children to access treatment in 2018 through 2021, however only 1.9 million children were reported to have received anti-tuberculosis treatment during that time. Hence, there is a substantial treatment gap of children that never access care for their tuberculosis disease.
Tuberculosis as a Bread-and-Butter Diagnosis in Sick Children in Sub-Saharan Africa
I am a paediatrician working in a busy public sector hospital in South Africa, one of the 30 high burdened countries with tuberculosis according to the WHO.(World Health Organization 2022b) South Africa has a three-fold burden of tuberculosis in terms of having high incidence rates of the disease, coupled with a high burden of co-infection with HIV in people suffering from tuberculosis, and a high prevalence of drug-resistant (including multi-drug resistant) tuberculosis.(World Health Organization 2022b)
Tuberculosis is a masterful masquerader and is frequently considered as part of the differential diagnosis in children hospitalised with pneumonia in high burden settings. In the Pneumonia Etiology Research for Child Health (PERCH) Study, a multi-site case-control study which investigated the organisms causative of WHO-defined severe pneumonia, M. tuberculosis was implicated as the primary cause of acute pneumonia in 6% of children under-5 years of age in low-middle income countries in sub-Saharan Africa and South East Asia. (Pneumonia Etiology Research for Child Health Study Group 2019)
The gold standard diagnostic test for tuberculosis is through isolation of the causative organism in clinical specimens; however, these specimens are difficult to obtain, especially from small children with pulmonary tuberculosis who are unable to produce sputum samples. Prolonged turn-around times on test results impact negatively on clinician decisions to start anti-tuberculosis treatment in children with suspected tuberculosis, and children are frequently initiated onto therapy pre-emptively. As M. tuberculosis is a slow-growing organism, it may take up to 6 weeks before culture results are finalised. Furthermore, culture of specimens has poor sensitivity (low detection rates, even in children with clinical features highly suggestive of tuberculosis) and only 20% to 40% of children with a clinical diagnosis of tuberculosis have microbiological confirmation of disease. (Moore et al. 2017; Gunasekera et al. 2021)
Clinicians often rely on a constellation of features to diagnose tuberculosis in children. The medical history and physical examination are key elements of the diagnostic work-up. If a young child is known to have a family member with tuberculosis, a diagnosis of tuberculosis must always be considered. “Classical symptoms” of tuberculosis include prolonged, non-resolving cough, weight loss, poor appetite, persistent fever, and profuse night sweats; however, these are often not present in very young children with tuberculosis. In a study from South Africa, 77% of children with culture-confirmed pulmonary tuberculosis had a cough duration of less than 10 days, indicating that the conventional impression of a long duration of cough may not always be suitable for children with tuberculosis. (Moore, Klugman, and Madhi 2010)
Chest X-rays in Children with Tuberculosis
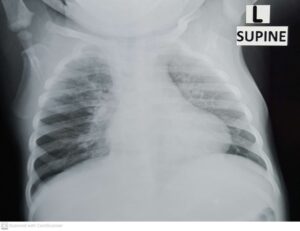
No chest X-ray features are specific for tuberculosis; however, certain findings are suggestive, particularly when taking the clinical history and examination into consideration. Shown here are three chest X-rays from children diagnosed with pulmonary tuberculosis in the Paediatric Department where I work. The first image (labelled “Primary tuberculosis”) shows a full shadow on the right side of the heart, indicative of enlarged mediastinal lymph nodes as well as extensive infiltrates in the left lung’s upper lobe: this X-ray was obtained from a 17-month-old girl hospitalized with pneumonia. Treating clinicians decided to start anti-tuberculosis therapy based on the impression of enlarged lymph nodes, which is a classical finding in primary tuberculosis. Tuberculosis microbiology test results were pending at the time of writing this article, illustrating how clinicians frequently initiate therapy prior to confirmation of the disease through microbiological testing.
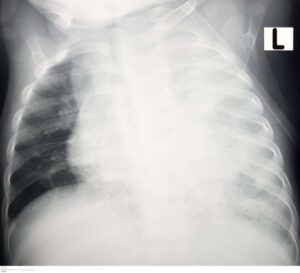
The second X-ray (labelled “TB pneumonia”) shows extensive pneumonia changes in the left lung, as well as the impression of lymph node enlargement in the upper mediastinum in a 9-month-old boy. There is also narrowing of the right main bronchus, indicating that enlarged lymph nodes are compressing the airway. This child’s tuberculosis culture result was positive, and the X-ray gives an example of the extent of disease that may be present in children with tuberculosis pneumonia.
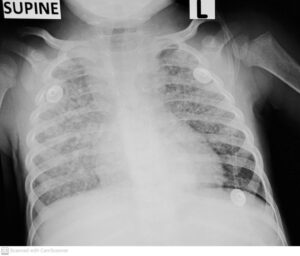
The third X-ray (labelled “Miliary tuberculosis”) shows the classical pattern of miliary tuberculosis involving both lungs in a 16-month-old boy hospitalised with severe weight loss and diarrhoea. The lung fields are infiltrated with millet seed sized nodules, and there is fullness of the mediastinum above the heart, once again indicating the presence of enlarged lymph nodes in the chest. This child also had culture-confirmed tuberculosis, with M. tuberculosis growing on blood culture, and detected using molecular testing of respiratory specimens.
Big Data and Clinical Algorithms
Given the difficulties in assigning a diagnosis of tuberculosis in children, based on microbiology and radiographic findings alone, there is renewed interest in developing clinical decision-making algorithms to assist healthcare workers decide which children warrant anti-tuberculosis therapy. Paediatric tuberculosis clinical scoring systems were developed in the 1980s but were not widely validated and fell out of favour in the past decade. No mention is made of a tuberculosis clinical scoring system to aid the diagnosis of the disease in children in the South African Paediatric TB Guideline document from 2013. (South African Department of Health 2013)
New approaches to data analysis, using large data sets from prospective studies evaluating children with presumed tuberculosis, are being used to develop data-driven clinical algorithms for pragmatic use in primary health care settings, as well as for children hospitalised with presumed tuberculosis.(Gunasekera et al. 2021) Features such as cough duration, fever, poor weight gain, lethargy and enlargement of the liver on clinical examination, are assigned individual scores which when summed together provide a combined score to direct healthcare workers through a diagnostic work flow that culminates in initiation of anti-tuberculosis treatment if criteria are met to do so.
Although clinical algorithms are often context-specific, reflecting the ways in which tuberculosis manifests in children in varying geographic settings with differing burdens of tuberculosis, they show promise for expediting access to anti-tuberculosis treatment for children. Furthermore, features on history and clinical examination count towards a clinical diagnosis of the condition which is particularly useful in peripheral clinics in low-income settings where X-ray facilities and access to laboratory diagnostics may be scarce.
The WHO currently endorses the use of integrated treatment decision algorithms to assist in the diagnosis of pulmonary tuberculosis in children (albeit a conditional recommendation with very low quality of evidence).(World Health Organization 2022a)
New Treatment Strategies
Conventional treatment for drug-susceptible tuberculosis consists of a 6-month course of anti-tuberculosis medications. Daily adherence to this long course of therapy is burdensome on families and children and may not be warranted in young children with paucibacillary tuberculosis disease. The effectiveness of tuberculosis treatment using a 4-month regimen in selected paediatric patients under 16 years of age was demonstrated in the SHINE Trial,(Turkova et al. 2022) and offers the hope of treatment shortening which is anticipated to translate into better adherence to therapy with better cure rates, and unburdening of saturated national tuberculosis programmes.
The WHO strongly recommends the use of the 4-month course of treatment for children under 16 years of age with non-severe, treatment susceptible tuberculosis, (World Health Organization 2022a) and national treatment guidelines are being revised to reflect this recommendation.
Strategies to evaluate the effectiveness of short-course anti-tuberculosis treatment in operational settings will need to be implemented to monitor outcomes to treatment, cost-effectiveness of care, and performance of treatment programmes once 4-month anti-tuberculosis therapy becomes standard of care for the management child tuberculosis suspects.
Concluding Remarks
The landscape of childhood tuberculosis looks both bleak and rosy. Bleak in terms of the burden of disease, exacerbated by the impact which COVID-19 had on national tuberculosis programmes globally: it will take considerable investment in terms of funding support and optimisation of treatment programmes to reverse the damage which COVID-19 had on tuberculosis surveillance and management strategies, particularly in low-income settings. Rosy because of the new approaches to diagnosis and treatment which place stronger emphasis on clinical history taking and physical examination - rather than expensive microbiological testing and chest X-ray - in the diagnostic pathway used by clinicians treating children with suspected tuberculosis.
On this World TB Day (24th March 2023), spare a thought for the millions of people affected by tuberculosis, accessing care through treatment programmes worldwide, and for their families and the healthcare workers that care for them. Spare a thought for the vulnerable children impacted by this disease daily, particularly in in low-income settings such as sub-Saharan Africa. And let us hope that, through a consolidated effort of optimisation of management and prevention pathways informed through modern collaborative research efforts, we will realise a world free of tuberculosis during our lifetimes.
By David P Moore, ISID Emerging Leader, South Africa
Associate Professor, Paediatric Infectious Diseases
Department of Paediatrics & Child Health
Chris Hani Baragwanath Academic Hospital
University of the Witwatersrand
