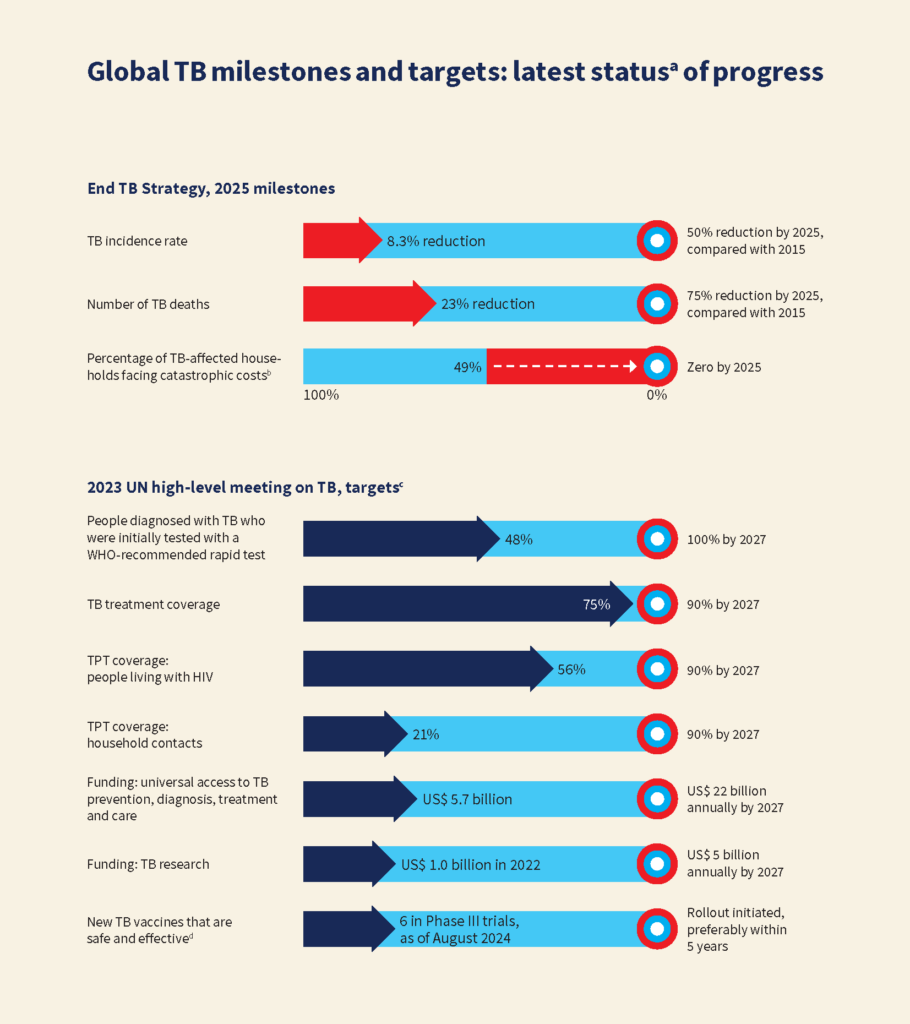
TB, the world’s leading cause of death due to a single infectious disease, was responsible for 10.8 million new cases and 1.25 million deaths in 20231. It continues to pose a significant public health concern, predominantly in low- and middle-income countries. World TB day, celebrated on the 24th of March every year, commemorates the date in 1882 when Dr. Robert Koch discovered this bacterium for the first time and serves as a reminder of the urgent need for innovative strategies, a call towards developing and applying ground-breaking approaches to eliminate TB and move towards a healthier global population.
Here some latest updates in TB diagnostics and management:
"Diagnosing TB – From Six Weeks to 90 Minutes! From One Drug to Nine Drugs!"
In addition to Xpert MTB/Rif (Cepheid) and Xpert MTB/Rif Ultra (Cepheid) recommended as the initial tests to diagnose pulmonary TB, WHO now recommends the use of Targeted Next Generation Sequencing directly from respiratory samples in bacteriologically confirmed TB to detect resistance against isoniazid, fluoroquinolones, bedaquiline, linezolid, clofazimine, pyrazinamide, ethambutol, amikacin and streptomycin; in its latest guideline2. In addition to the provision of the WHO Catalogue of mutations in Mycobacterium tuberculosis3 to perform genomic mutational analysis, this is a huge step forward to diagnose drug resistance in the shortest possible time. While phenotypic methods remain the gold standard for TB diagnosis today, with these diagnostic advances, genotypic methods may soon take over the crown.
"Shorter = Longer"
While TB diagnostics advance by leaps and bounds, TB therapeutics are not far behind. With several new trials reporting the non-inferiority of shorter regimens compared to traditional regimens, the recommendation for a two-month4 and a six-month5-9 regimen for drug-susceptible and drug-resistant pulmonary TB, respectively, seems to be just around the corner. The shortening of TB regimens, when instituted on a programmatic scale, would have a significant positive impact on public health policies, improve patient compliance, and significantly reduce pill burden, drug toxicities, and treatment-associated costs.
"The Reckoning: Phase 3 Trial for the M72/ASO1E Begins"
The much-awaited Phase 3 trial for the M72/ASO1E TB candidate vaccine, estimated to enroll over 20000 participants from South Africa and South-East Asia, enrolled the first participant in March 202410. This is the largest TB vaccine trial ever conducted, thanks to the generous donation by the Bill and Melinda Gates Foundation and the Wellcome Trust. This vaccine demonstrated an unprecedented 50% efficacy in preventing clinical TB in a phase 2B trial11. If successful in Phase 3 trials, it would be the first vaccine in 100 years to prevent Pulmonary TB. The World Health Organization estimates that over 25 years, that level of protection could save 8.5 million lives, prevent 76 million new TB cases, and save $41.5 billion for TB-affected households12.