GUIDE TO INFECTION CONTROL IN THE HEALTHCARE SETTING
PREPARING THE PATIENT FOR SURGERY
Authors: Helen Giamarellou, MD, PhD; Anastasia Antoniadou MD, PhD
Chapter Editor: Victor D. Rosenthal, MD, CIC, MSc
Print PDF
KEY ISSUES
- Surgical site infections (SSIs) are preventable and epidemiologically important, affecting up to one third of patients who had surgery in low- and middle-income countries1-8and being the second most common healthcare-associated infection in Europe and the USA. Prevention requires measures before, during, and after surgery.
- Appropriate organ function support, skin preparation, antimicrobial prophylaxis and wound care, decrease the incidence of both incisional and deep infections (organ or space) after certain operations.
KNOWN FACTS
New guidelines have been issued by WHO (2016) and CDC (2017). Decreasing the risk of surgical site infections (SSIs) and strongly recommended are: a preoperative shower, decolonization of patients with known nasal carriage of Staphylococcus aureus (especially in cardiothoracic and orthopaedic surgery), avoiding hair removal or, if this is absolutely necessary, removal with a clipper, surgical site skin preparation with alcohol-based antiseptics in the operating room, a single preoperative dose of a first- or second-generation cephalosporin within 120 minutes before incision (considering the half-time of the antibiotic) and intraoperative organ support with normothermia, hyperoxygenation, and intensive blood glucose control (<200 mg/dl). Regrettably, more than one postoperative doses of prophylaxis are generally administered in several medical centers leading to excess cost and the emergence of multiresistant bacteria.
CONTROVERSIAL ISSUES
- Assessment of risk factors in clean and laparoscopic operations requires more studies.
- Weight-based dosing for antimicrobial prophylaxis in obese patients must be clarified.
- The protocols for screening for nasal aureus carriage before surgery and decolonization with mupirocin must be precisely defined.
- Rectal screening for extended-spectrum beta-lactamases (ESBL) or other multidrug-resistant (MDR) pathogens according to risk factors and the impact to SSI incidence and outcome should be clarified.
DEFINITIONS
- Surgical site infections (SSIs) are infections of the surgical site incision or organ or space, occurring after surgery. The superficial (incisional) infections involve only the skin and subcutaneous tissues while deep (organ or space) infections involve at least muscle and fascial layers. Incisions may be contaminated by the patient’s own normal flora or by flora from the environment, including the operative team. Correct surveillance of SSIs extends to 30 days following surgery. In the case of implants, surveillance is extended from 90 days (USA) for up to 1 year (Europe, ECDC).
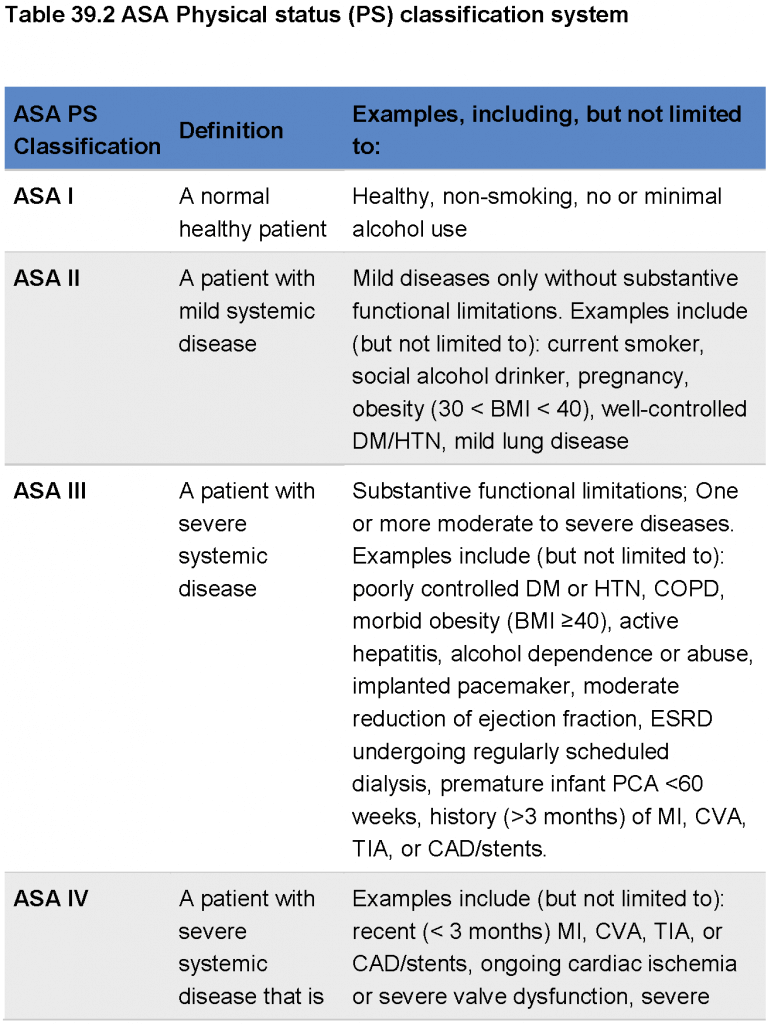
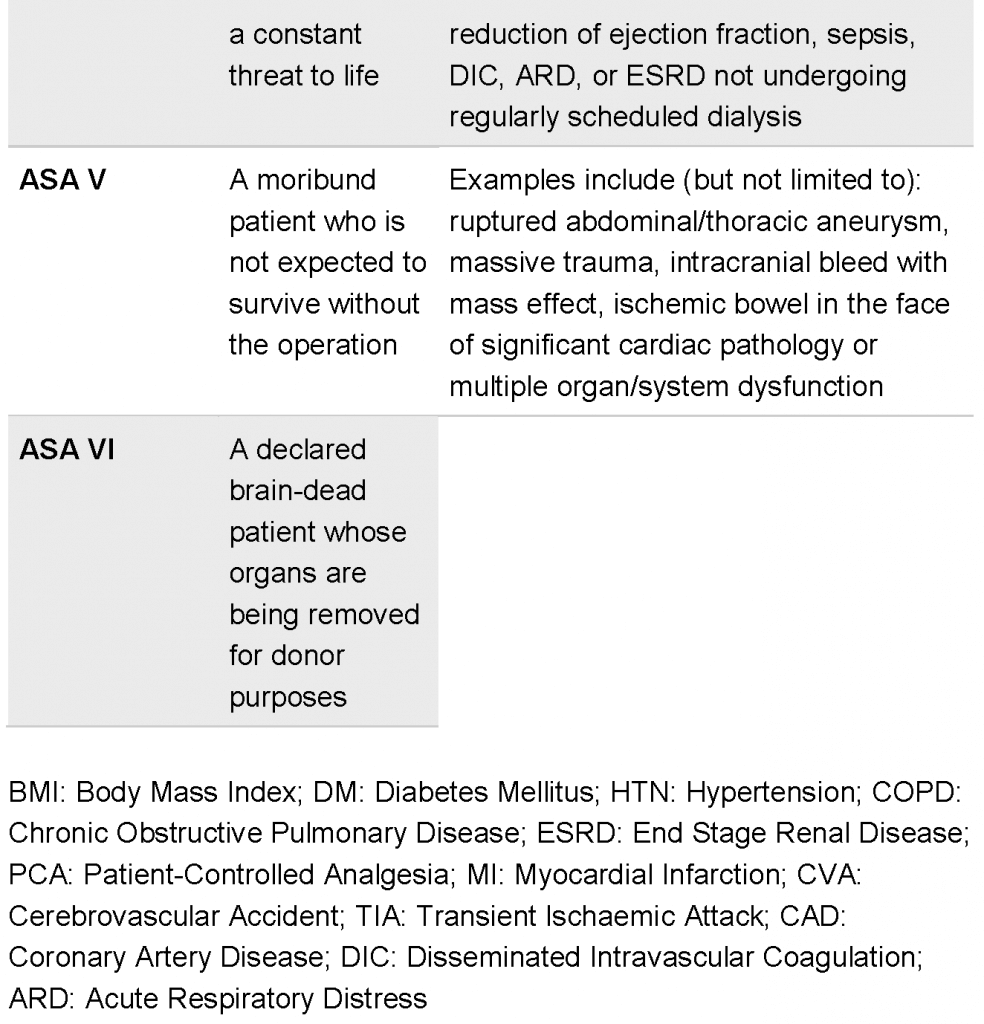
- The surgical wound classification system was established based on the exposure of the incision to bacterial contamination (see Table 39.1). Infection was reported in 3.3% of clean wounds, in 10.8% of clean-contaminated, in 16.3% of contaminated, and in 28.6% of dirty wounds. The risk for SSI is multifactorial, including patient status and comorbidities, clinical practice, surgery, and wound class characteristics. Efforts for indexes to estimate the patient’s risk for SSI have been made, for example the NNIS (Nosocomial Infections Surveillance System) risk index which includes 3 factors: (a) a contaminated or dirty wound class (1 point); (b) a high preoperative risk as defined by the American Society of Anesthesiologists (ASA) preoperative assessment score (seeTable 39.2)of 3 or more (1 point); and (3) duration of operation exceeding the 75th percentile for a given procedure (1 point). With zero points the risk for SSI is estimated to be 1.5% and for a sum of 3 points 13%. Malnutrition, advanced age, increased patient dependence, obesity, diabetes mellitus, renal insufficiency, cirrhosis, coexisting remote body-site infections, recent surgical procedure, increased length of preoperative hospitalization, known colonization with methicillin-resistant aureus (MRSA) or vancomycin-resistant enterococci (VRE), placement of foreign bodies, malignancy, and the use of steroids or immunosuppressive drugs represent additional risk factors for SSI, as evident by systematic reviews.
- The heavy burden of SSI in low and middle-income countries.1-8is illustrated in findings of a cohort, prospective, multicenter surveillance study on SSIs conducted by the International Nosocomial Infection Control Consortium (INICC) in 82 hospitals of 66 cities in 30 countries (Argentina, Brazil, Colombia, Cuba, Dominican Republic, Egypt, Greece, India, Kosovo, Lebanon, Lithuania, Macedonia, Malaysia, Mexico, Morocco, Pakistan, Panama, Peru, Philippines, Poland, Salvador, Saudi Arabia, Serbia, Singapore, Slovakia, Sudan, Thailand, Turkey, Uruguay, and Vietnam). SSI rates were significantly higher for most types of surgical procedures analyzed in INICC hospitals compared with CDC-NHSN (National Healthcare Safety Network) data, including the rates of SSI after hip prosthesis (2.6% vs. 1.3%; relative risk [RR], 2.06 [95% confidence interval (CI), 1.8-2.4]; P <.001), coronary bypass with chest and donor incision (4.5% vs. 2.9%; RR, 1.52 [95% CI, 1.4-1.6]; [P <.001); abdominal hysterectomy (2.7% vs. 1.6%; RR, 1.66 [95% CI, 1.4-2.0]; P <.001); exploratory abdominal surgery (4.1% vs. 2.0%; RR, 2.05 [95% CI, 1.6-2.6]; P <.001); ventricular shunt, 12.9% vs. 5.6% (RR, 2.3 [95% CI, 1.9-2.6]; P <.001, among others.8
SUGGESTED PRACTICE
General Principles
Patient Preparation for Surgery
Preparation of patients for surgery aiming at preventing postoperative SSI is based on appropriate skin care, and antimicrobial prophylaxis. Additional recommended measures are:
- Appropriate treatment of remote infections before elective operations.
- Intensive control of blood glycose levels(<200 mg/dl or even <180 mg/dl) perioperatively both in diabetics and not diabetics.
- Perioperative oxygenation in adult patients undergoing general anaesthesia with endotracheal intubation for surgical procedures, who should receive an 80% fraction of inspired oxygen (FiO2) intraoperatively and, if feasible, in the immediate postoperative period for 2-6 h.
- Maintenance of normothermia in the operating room either by the use of warming devices or blankets.
- Decolonization of known nasal carriage of aureusespecially in orthopaedic and cardiothoracic surgery with intranasal 2% mupirocin ointment for 5 days, with or without a combination of a chlorhexidine body wash.
- Mechanical bowel preparation in colorectal procedures, combined with oral neomycin sulfate plus erythromycin base or metronidazole, which should be given the day prior to surgery, in addition to preoperative IV antimicrobial prophylaxis. Mechanical bowel preparation should not be used alone.
Surgical Site Preparation and Care
Decontamination of the skin preoperatively is very important to prevent wound infection, particularly in clean procedures. A shower before surgery with an antimicrobial or plain soap is recommended. Hair removal at the operative site is discouraged since existing evidence shows that patients with no hair removal may have even lower rates of SSIs; when considered absolutely necessary it must be done with clippers preferably before operation. Skin preparation in the operating room at the surgical site should be performed by trained personnel using an alcohol-based antiseptic solution based on chlorhexidine gluconate. Antimicrobial skin sealants after skin preparation, or plastic adhesive incise drapes should not be used. Sterile drapes, non-woven disposable or woven reusable are suggested. Triclosan-coated sutures are suggested to be used in all types of surgery. Irrigation of incision with antibiotics before closure must be avoided as also the use of advanced dressings (e.g., silver impregnated) since they provide no benefit compared to standard dressings. If resources are permissive, prophylactic negative-pressure wound therapy is suggested in high-risk wounds (e.g., poor tissue perfusion, bleeding or hematoma, dead space, intraoperative contamination). Antimicrobial agents (e.g., ointments, solutions, or powders) should not be applied to the surgical incision for the prevention of SSIs. Laminal air flow ventilation systems for patients undergoing total arthroplasty should not be used.
Suggested Practice in Antimicrobial Prophylaxis
- A single, full therapeutic dose of an antibiotic should be given intravenously within 120 minutes before surgical incision (30-120 minutes considering the half-life of the antibiotic) to ensure effective tissue concentrations throughout the operative period. Prophylaxis should not be extended beyond 24 hours following surgery. An exception to this rule is cardiac surgery where prolongation of antimicrobial prophylaxis for 24-48 hours seems to be beneficial. Antibiotics are effective when given before inoculation of bacteria at the surgical site, whereas they are ineffective if given 3 to 4 hours after the surgical incision. Continuing prophylaxis until all indwelling drains and intravascular catheters are removed is strongly discouraged.As long as adequate serum and tissue drug levels against probable pathogens are maintained during the operation, a single dose is as effective as multiple doses. Current limited evidence does not support a modification in dosing for obese patients.
- The selection of the appropriate drug should be based on the bacteria most likely to cause infection in each situation, its safety profile, as well as the local resistance surveillance patterns. A single drug should be used, whenever possible. Cephalosporins, particularly and particularly cefazolin is ideal for prophylaxis because of its broad spectrum of activity, the moderately long serum half-life, low toxicity, ease of administration, and low cost. In clean-contaminated cases without entry into the gastrointestinal tract and in clean operations involving the surgical placement of foreign material (e.g., heart valves, vascular grafts, orthopedic hardware, etc.), or whenever risk factors for SSI coexist, cefazolin alone should be administered. In clean contaminated operations with entry into the gastrointestinal tract as well as in penetrating abdominal trauma or primary appendectomy, cefazolin plus an agent active against anaerobes like metronidazole as well as cefotetan or cefoxitin as single agents, should be used. Administration of antibiotics in contaminated and dirty operations is considered therapy and not prophylaxis. Third-generation cephalosporins are more costly and promote the emergence of resistant strains. In general, they should not be used routinely for prophylaxis.
- In colorectal surgery and in institutions where there is increasing resistance to first and second generation cephalosporins among Gram-negative isolates from SSIs, ceftriaxone plus metronidazole should be preferred over ertapenem. For patients with beta-lactam allergies, metronidazole or clindamycin plus an aminoglycoside or a fluoroquinolone or aztreonam could replace as above the suggested regimens.
- Since staphylococci are the major threat in infected prostheses, vancomycin instead of cefazolin should be used in institutions with a high predominance of methicillin-resistant strains (>15-20%) as well as in beta-lactam allergic patients. Because of prolonged infusion time required for vancomycin (1h) it should be administered within 120 min before surgical incision.
- In the case of excessive blood loss (>1,5 Lt), or whenever the duration of operation exceeds 2 half-lives of the preadministered antibiotic(s), intraoperative redosing should be given. The redosing interval should be measured from the time of administration of the preoperative dose, not from the beginning of the procedure.
- In laparoscopic biliary tract procedures since some factors increasing the risk for SSI cannot be determined before the procedure (e.g., gallbladder empyema, perforation or infection, prolongation of procedure >60 minutes), it may be reasonable to give a single dose of antimicrobial prophylaxis to all patients.
- With the exception of ophthalmic procedures, topical administration of antibiotics as prophylaxis, based on their lack of efficacy and the possibility of adverse reactions, is not recommended.
SUGGESTED PRACTICE IN UNDER-RESOURCED SETTINGS
- In 2016 WHO issued Global guidelines for the prevention of surgical site infection with a panel of experts from all 6 WHO regions. These guidelines for the pre-, intra- , and postoperative patient care were elaborated according to the best available scientific evidence and expert consensus, with the aim to ensure high-quality care for every patient, irrespective of the resources available (http://www.who.int/gpsc/ssi-guidelines/en/). Recommendations rated as “strong” in these guidelines are considered to be adaptable for implementation in most (if not all) situations, and patients should receive the intervention as the course of action. For “conditional” recommendations, a more structured decision-making process should be undertaken, considering stakeholder consultation, availability of resources, patients’ and healthcare professionals’ preferences. Most of the recommendations presented above are included in those “strongly” recommended by WHO.
There is no doubt that appropriate antibiotic prophylaxis along with patient and surgical site preparation reduce morbidity and costs by preventing surgical site infections. However, it should be emphasized that antibiotic overuse and misuse for surgical prophylaxis accounts for as many as half of all antibiotics costs prescribed in U.S. hospitals and contributes to the emergence of multidrug-resistant microorganisms particularly whenever the one single preoperative dose is exceeded. Based on the importance of the application of correct perioperative antibiotic prophylaxis as well as the appropriate preparation of patients for surgery, it has been recently suggested that hospitals should establish a multidisciplinary team including surgeons, anaesthesiologists, nurses, pharmacists, infection control specialists, and clinical microbiologists, who should develop and implement a relevant bundle protocol.

REFERENCES
- Bratzler DW, Dellinger EP, Olsen KM, et al. Clinical Practice Guidelines for Antimicrobial Prophylaxis in Surgery. Am J Health-Syst Pharm 2013; 70(3): 195–83. doi: 10.2146/ajhp120568.
- Classen DC, Evans RS, Restomic SL, et al. The timing of prophylactic administration of antibiotics and the risk of surgical wound infection. N Engl J Med1992; 326(5):281–6.
- European Center for Disease Prevention and Control Technical Report: Systemic Review and Evidence-Based Guidance on Perioperative Antibiotic Prophylaxis. Stockholm: ECDC; 2013 Catalogue number TQ-01-13-279-EN-C.
- Korol E, Johnston K, Waser N, et al. A Systematic Review of Risk Factors Associated with Surgical Site Infections among Surgical Patients. PloS One 2013; 8(12):1–9. doi: 10.1371/journal.pone.0083743.
- Allegranzi B, Bischoff P, de Jonge S, et al. New WHO Recommendations on Preoperative Measures for Surgical Site Infection Prevention: An Evidence-Based Global Perspective. Lancet Infect Dis 2016; 16(12):e276–87. doi: 10.1016/S1473-3099(16)30398-X.
- Allegranzi B, Zayed B, Bischoff P, et al. New WHO Recommendations on Intraoperative and Postoperative Measures for Surgical Site Infection Prevention: An Evidence-Based Global Perspective. Lancet Infect Dis 2016; 16(12):30402–9.
- Berríos-Torres SI, Umscheid CA, Bratzler DW, et al. Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection, 2017. JAMA Surg 2017; 152(8):784–91. doi: 10.1001/jamasurg.2017.0904.
SUGGESTED REFERENCES IN UNDER-RESOURCED SETTINGS
- Viet Hung N, Anh Thu T, Rosenthal VD, et al. Surgical Site Infection Rates in Seven Cities in Vietnam: Findings of the International Nosocomial Infection Control Consortium. Surg Infect (Larchmt). 2016; 17(2):243–9. doi: 10.1089/sur.2015.073.
- Richtmann R, Siliprandi EM, Rosenthal VD, et al. Surgical Site Infection Rates in Four Cities in Brazil: Findings of the International Nosocomial Infection Control Consortium. Surg Infect (Larchmt) 2016; 17(1):53–7. doi: 10.1089/sur.2015.074.
- Singh S, Chakravarthy M, Rosenthal VD, et al. Surgical Site Infection Rates in Six Cities of India: Findings of the International Nosocomial Infection Control Consortium (INICC). Int Health 2015; 7(5):354–9. doi: 10.1093/inthealth/ihu089.
- Ramirez-Wong FM, Atencio-Espinoza T, Rosenthal VD, et al. Surgical Site Infections Rates in More Than 13,000 Surgical Procedures in Three Cities in Peru: Findings of the International Nosocomial Infection Control Consortium. Surg Infect (Larchmt) 2015. 16(5):572–6. doi: 10.1089/sur.2014.201.
- Leblebicioglu H, Erben N, Rosenthal VD, et al. Surgical Site Infection Rates in 16 Cities in Turkey: Findings of the International Nosocomial Infection Control Consortium (INICC). Am J Infect Control 2015; 43(1):48–52. doi: 10.1016/j.ajic.2014.09.017.
- Portillo-Gallo JH, Miranda-Novales MG, Rosenthal VD, et al. Surgical Site Infection Rates in Four Mexican Cities: Findings of the International Nosocomial Infection Control Consortium (INICC). J Infect Public Health 2014; 7(6):465–71. doi: 10.1016/j.jiph.2014.07.015
- Alvarez-Moreno C, Perez-Fernandez AM, Rosenthal VD, et al. Surgical Site Infection Rates in 4 Cities in Colombia: Findings of the International Nosocomial Infection Control Consortium (INICC). Am J Infect Control. 2014; 42(10):1089–92. doi: 10.1016/j.ajic.2014.06.010.
- Rosenthal VD, Richtmann R, Singh S, et al. Surgical Site Infections, International Nosocomial Infection Control Consortium (INICC) Report, Data Summary of 30 Countries, 2005-2010. Infect Control Hosp Epidemiol 2013; 34(6): 597–604.
