GUIDE TO INFECTION CONTROL IN THE HEALTHCARE SETTING
LABORATORY AREAS
Authors: Morgan A. Pence, PhD
Chapter Editor: Gonzalo Bearman, MD, MPH, FACP, FSHEA, FIDSA
Print PDF
KEY ISSUES
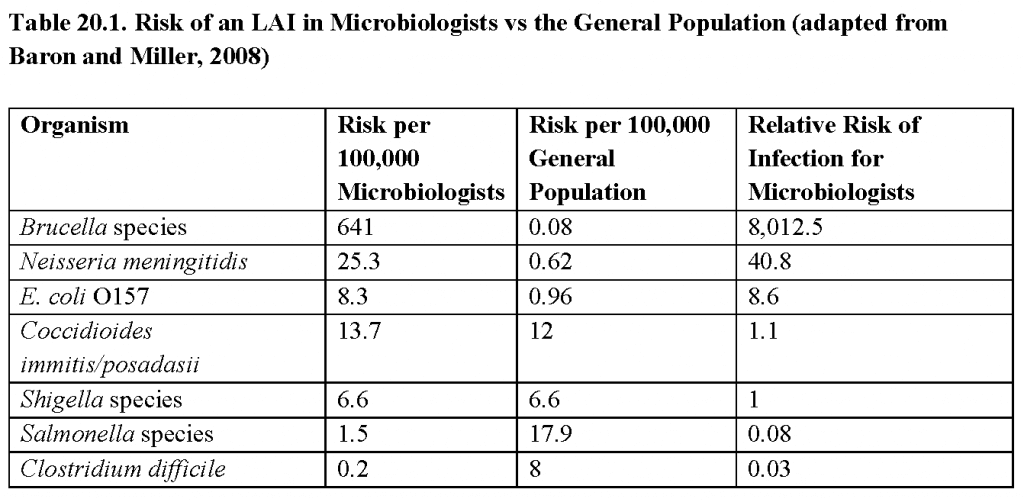
- Laboratory workers are exposed to a variety of potential occupational health risks that include infectious materials and cultures. Laboratory-acquired infections (LAIs) are defined as all infections acquired through laboratory activities, regardless of their clinical or subclinical manifestations. Biosafety guidelines have evolved from the efforts of the microbiological and biomedical communities to reduce LAIs. The actual risk of a laboratory-acquired infection is difficult to measure because there is no systematic reporting at a government or professional society level to monitor the number of laboratory workers that acquire infections associated with the workplace. Reports in the literature have all been survey-based. Sulkin and Pike reported on more than 4,000 laboratory-associated infections between 1949 and 1974, with a mortality of 4.1%. During those years, brucellosis, Q fever, typhoid fever, and hepatitis were the most common causes of LAIs. More recent surveys have revealed a shift in the pattern of LAIs from the early collective studies. A 2002-2004 survey of clinical laboratory directors revealed that approximately one-third of laboratories reported at least one LAI. Shigellosis, brucellosis, and salmonellosis were the three most common LAIs followed by Staphylococcus aureus, Neisseria meningitidis, Escherichia coliO157:H7, Coccidioides immitis/posadasii, Clostridium difficile,and Bacillus anthracis. The relative risk of infection for microbiologists compared to the general population ranges from 0.03 to 8,000, depending on the pathogen(see Table 20.1).
- To minimize the risk of LAIs, laboratories must develop a program that encompasses a combination of engineering controls (including laboratory design), safe laboratory practices, employee education, personal protective equipment (PPE), and medical measures that include surveillance, risk assessment, vaccination, and post-exposure prophylaxis. The development of such programs to minimize risks associated with the handling and disposal of infectious agents is based on an understanding of the pathogenicity of the agent, host susceptibility, source of infection, and the method of transmission of the infectious agent. Most risks from biological hazards can be reduced through the use of appropriate microbiological procedures and techniques, containment devices and facilities, and protective barriers.
PRINCIPAL ROUTES OF LABORATORY TRANSMISSION
- Surveys in the US between 1978 and 1986, reported an annual incidence of 3 to 3.5 infections per 1,000 laboratory employees per year. The present incidence of LAIs is unknown; however, Wilson and Reller estimated that the current annual rate of LAIs in the U.S. is approximately 1 to 5 infections per 1,000 employees.
- Clinical diagnostic laboratories accounted for 45% of all laboratory-acquired infections. Laboratory workers, especially those in microbiology, are at greater risk of becoming infected than the general population.
- The causative source, procedure, or breach in technique cannot be identified in approximately 50% of LAIs.
- There is a lack of evidence-based research and publications focused on biosafety, specifically studies documenting safe practices in the day-to-day operations of diagnostic laboratories.
- In 2008, the Centers for Disease Control and Prevention (CDC) convened a Blue Ribbon Panel to review laboratory biosafety in diagnostic laboratories. The Clinical and Laboratory Standards Institute (CLSI) has also published guidelines for the protection of laboratory workers from occupationally-acquired infections (M29-A4).
Inhalation of Aerosols and Droplets
- Pipetting, blenders, pouring, non-self-contained centrifuges, sonicators, vortex mixers, flaming a reusable loop, and catalase testing may generate airborne respirable size particles (<0.05 mm in diameter).
- Aerosol output and dose are impacted by procedure — aerosol burden with maximal aeration is approximately 200 times greater than aerosol burden with minimal aeration.
- Lyophilized cultures, dried materials on laboratory benches, and bacterial and fungal spores can act as droplet nuclei.
- Procedures and equipment that generate respirable size particles also generate larger size droplets (>0.1 mm in diameter). These larger size droplets settle out of the air, contaminating gloved hands, work surfaces, and possibly mucous membranes of the person performing the procedure.
Inoculation
- Parenteral inoculation of infectious materials with syringe needles or other contaminated sharps such as blades and broken glassware.
Contamination of Skin and Mucous Membranes
- Spills, sprays, and splashes into eyes, mouth, or nose and hand-to-face actions.
- Spills, sprays, and splashes onto skin cuts, abrasions, and dry, inflamed skin.
- Contaminated surfaces and equipment.
Ingestion
- Mouth pipetting and transfer of organisms to the mouth from contaminated items such as pencils or fingers.
- Consumption of food or drink in the laboratory.
- Accidental splashes into the mouth.
SUGGESTED PRACTICE
Risk Assessment
The assignment of an infectious agent to a biosafety level must be based on a risk assessment. Occupational risk assessment criteria are influenced by the type of manipulations or activities performed with the agent, the experience of the laboratory worker, and the infectious agent. Each task, procedure, or activity performed in the laboratory must be analyzed for its potential risk to the employee who performs the task. The international community has developed a common risk classification scheme in which infectious agents are categorized into four risk groups based on their relative risk to cause laboratory-associated infections. These groups are categorized based on particular characteristics of the infectious agent, such as pathogenicity, infectious dose, mode of transmission, host range, and availability of effective preventive measures and effective treatment. These risk groups were developed to help laboratories determine the best laboratory practices and environmental requirements for containment. Other factors associated with laboratory operations including specimen volume, potential for aerosol generation, quantity and concentration of infectious agents, agent stability in the environment, and type of work proposed should also be taken into consideration.
Levels of Containment
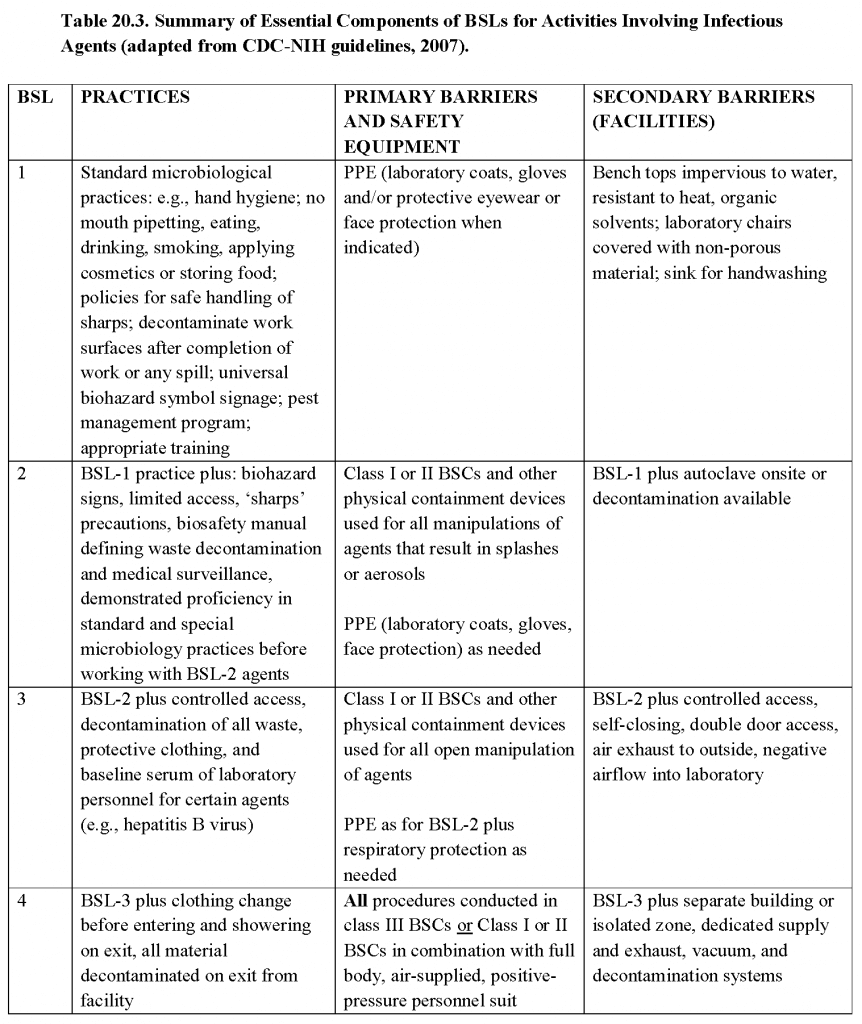
- In general, the strategy for minimizing the occupational exposure of laboratory workers to infectious agents is based on microorganism containment, which includes physical factors such as facility design and safety equipment, standard microbiological practices, and administrative controls. Microorganisms encountered and the procedures performed are stratified by risk. Primary risk criteria are used to define the four ascending levels of containment, biosafety levels (BSL) 1 through 4. Primary risk criteria include infectivity, severity of disease, transmissibility and the nature of the work being conducted. Each increasing BSL number implies increased occupational risk from exposure to a microorganism or performance of a procedure and thus is associated with more stringent control and containment practices:
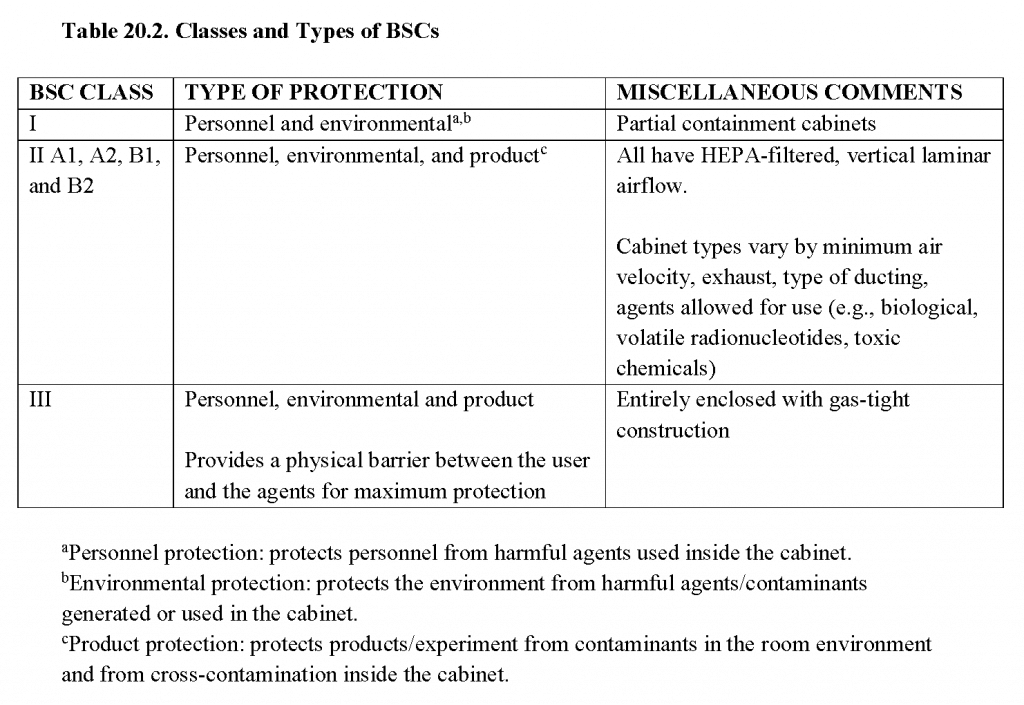
- Primary barriers:strict adherence to microbiological practices and techniques; use of PPE (e.g., gloves, masks, face shields, coats, gowns, respirators), safety centrifuge containers, sharps protection, and biological safety cabinets (BSCs; see Table 20.2)
- Secondary barriers: secondary barriers include facility design/separation of the laboratory from public access as well as availability of a decontamination facility, handwashing facilities, specialized ventilation, and/or airflow.
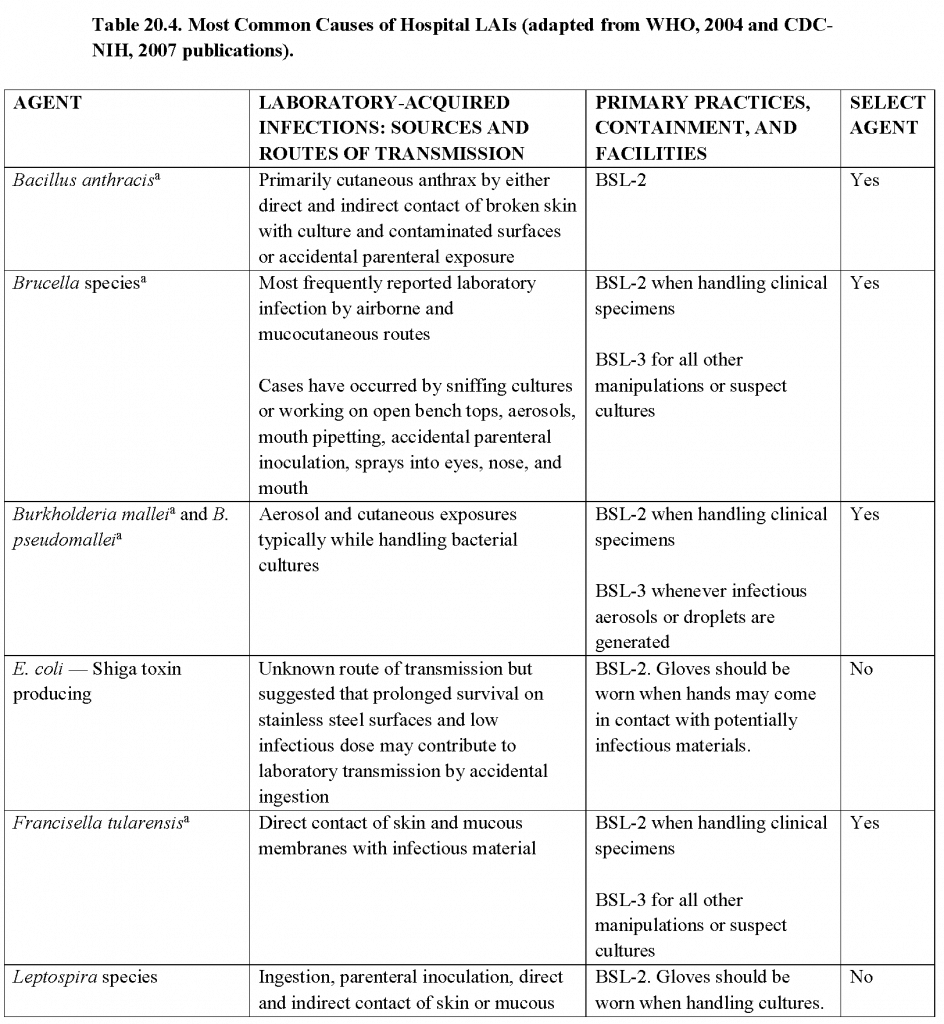
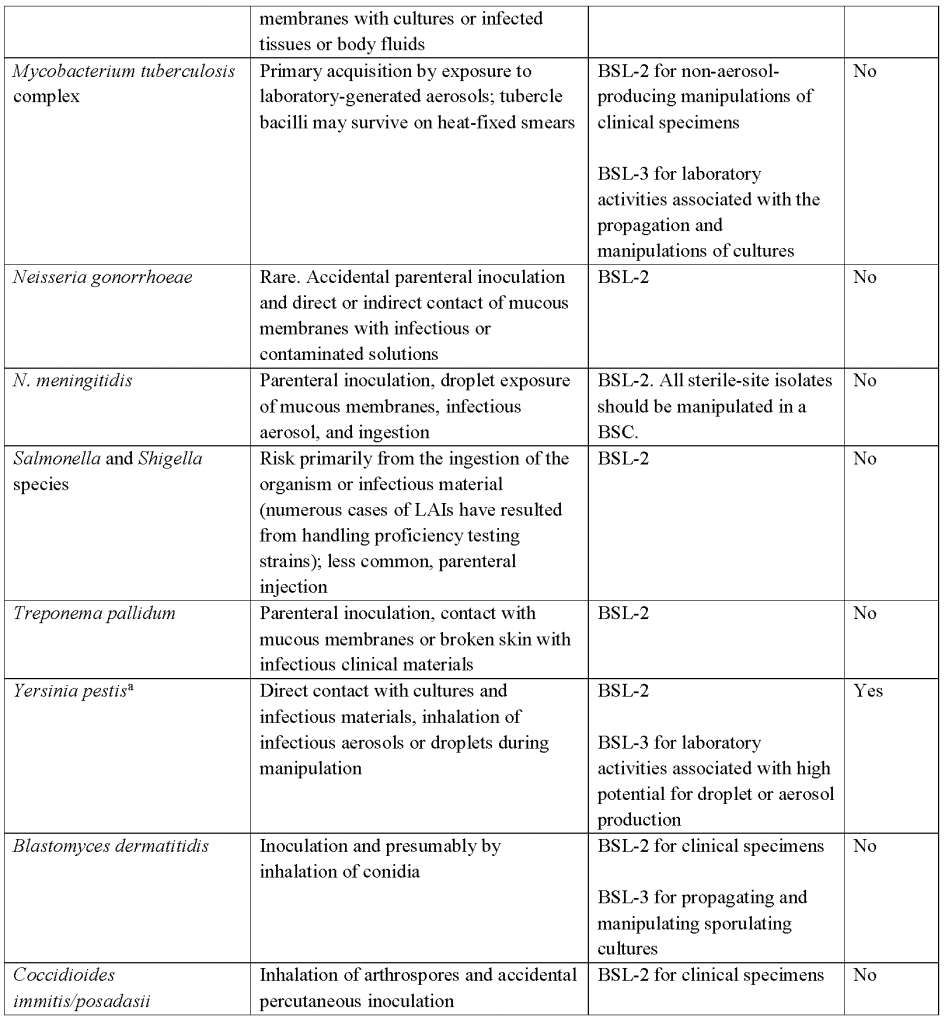
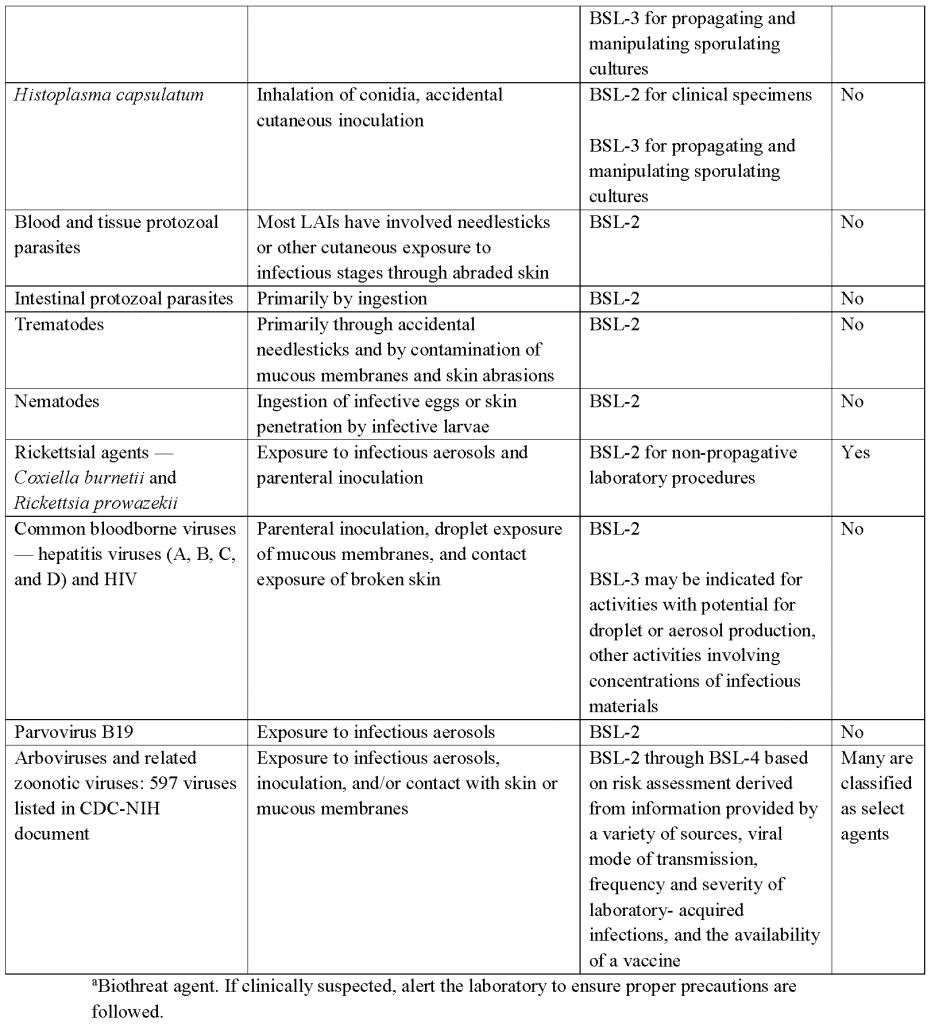
- A brief overview of practices and techniques, safety equipment, and facilities for recommended BSLs is shown in Table 20.3. In addition, the more common agents and those that could pose severe threats to animal or plant health (i.e., select agents) and their corresponding routes of transmission and primary practices, containment, and facilities in the laboratory are summarized in Table 20.4.In light of significant national and international events, biosecurity measures have been implemented and subsequently expanded to protect microbial agents from loss, theft, diversion. or intentional misuse. In the U.S., select agent regulations have led laboratory managers, scientists, scientific and institutional leaders, and others to implement and improve the security of biological agents and toxins within their facilities. Advisory recommendations for biosecurity programs are detailed in the National Institutes of Health (NIH)/CDC publication ’Biosafety in Microbiological and Biomedical Laboratories (BMBL)’, 5th Edition. Detailed information regarding biosafety levels recommended for specific bacteria, fungi, parasites, and viruses can be found in textbooks and a variety of websites such as:
- CDC Biosafety webpage: http://www.cdc.gov/od/ohs/biosfty/bmbl5/bmbl5toc.htm.
- WHO Biosafety Manual http://www.who.int/csr/resources/publications/biosafety/WHO_CDS_CSR_LYO_2004_11/en/.
- Canada’s Biosafety and Biosecurity webpage: http://www.phac-aspc.gc.ca/ols-bsl/lbg-ldmbl/index.html.
- Guidelines for Safe Work Practices in Human and Animal Medical Diagnostic Laboratories: http://www.cdc.gov/mmwr/preview/mmwrhtml/su6101a1.htm.
Administrative Elements of a Safe Clinical Laboratory
- Personal protective equipment program and procedures.
- Respiratory protection program.
- Biosafety, exposure control, and chemical hygiene plans that include procedures to address accidental spills of infectious organisms or release of infectious microorganisms into the laboratory or facility environment.
- Comprehensive plan for management and disposal of infectious waste including blood and blood products.
- Medical surveillance for infections that may result from exposure to agents encountered in the performance of routine duties or when early diagnosis reduces the risk of serious consequences of the infection (e.g., rickettsial infections)
- Safety manual, acknowledged and understood by employees, that includes the occupational risks and consequences of infection.
- Promotion of safety awareness through training programs and required adherence to safety procedures.
- Consistent observance by all workers of proven safety and microbiological practices.
- Documentation and reporting of all occupational injuries, illnesses, and incidents of potential exposure.
SUGGESTED PRACTICE IN UNDER-RESOURCED SETTINGS
- Some of the biosafety controls utilized in the United States and other high-income countries may not be available in low- and middle-income countries (LMICs). A consensus has not been reached with respect to suggested practices in LMICs. Some experts believe that LMICs should be held to the same standards as high-income countries, while other experts believe one set of standards is not feasible and therefore support the creation of a separate set of guidelines.
- Regardless of the location of a laboratory, a risk assessment should be performed to determine the risk of infection. Employee education, including training and competency, as well as primary barriers, proper hand hygiene, and strict adherence to proper procedures can prevent many LAIs.
SUMMARY
Even with advances in laboratory safety, LAIs still occur. Infections can occur via inhalation, inoculation, ingestion, and contamination of skin and mucous membranes. However, the exact causative source, procedure, or breach in technique cannot be identified in 50% of LAIs. Each individual laboratory should perform a risk assessment in which each task, procedure, or activity performed in the laboratory is analyzed for its potential risk to the employee. To most effectively prevent LAIs, laboratories should follow the recommended guidelines, including primary and secondary barriers, for the specific risk group assigned.
REFERENCES
- Baron, EJ, Miller M. Bacterial and Fungal Infections among Diagnostic Laboratory Workers: Evaluating the Risks. Diagn Microbiol Infect Dis. 2008; 60(3):241–6.
- CDC-NIH. Biosafety in Microbiological and Biomedical Laboratories (BMBL), 5th Edition. U.S. Department of Health and Human Services and the Institutes of Health, U.S. Government Printing Office, Washington, 2007.
- Miller JM, Astles R, Baszler T, et al. Guidelines for Safe Work Practices in Human and Animal Medical Diagnostic Laboratories: Recommendations of a CDC-Convened, Biosafety Blue Ribbon Panel. MMWR Suppl. 2012; 61(1):1–102.
- Clinical and Laboratory Standards Institute. Protection of Laboratory Workers from Occupationally Acquired Infections: Approved Guideline – Fourth Edition. CLSI document M29-A4E. 2014.
- Collins CH, Kennedy DA. Laboratory-Acquired Infections (4th Edition). Butterworth-Heineman, Oxford, England, 1999.
- Harding AL, Byers KB. Epidemiology of Laboratory-Associated infections. In: Biological Safety: Principles and Practices. (3rd Edition) Fleming DO, Hunt DL (Eds.). Washington, DC: ASM Press, 2000: 35–54.
- Health Canada. Laboratory Biosafety Guidelines (3rd Edition). Health Canada, Ottawa, Canada, 2004.
- Pike RM. Laboratory-Associated Infections: Summary and Analysis of 3921 Cases. Health Lab Sci 1976; 13(2):105–14.
- Pike RM. Laboratory-Associated Infections: Incidence, Fatalities, Causes and Prevention. Annu Rev Microbiol 1979; 33:41–66.
- Sewell DL. Laboratory-Associated Infections and Biosafety. Clin Microbiol Rev 1995; 8(3):389–405.
- Singh K. Laboratory-Acquired Infections. Clin Infect Dis 2009; 49(1):142–7. doi: 10.1086/599104.
- Sulkin SE, Pike R. Viral Infections Contracted in the Laboratory. N Engl J Med 1949; 241(5): 205–13.
- Wilson ML, Reller LB. Bennett and Brachman’s Hospital Infections, (6th Edition) Jarvis R (Ed.) Chapter 22: Clinical Laboratory-Acquired infections, 2013; 320–8.
- World Health Organization. Laboratory Biosafety Manual (3rd Edition), WHO, Geneva, Switzerland, 2004.