GUIDE TO INFECTION CONTROL IN THE HEALTHCARE SETTING
ANTIMICROBIAL STEWARDSHIP IN THE HOSPITAL SETTING
Authors: Jacob Pierce, MD, Dan Markley, DO, MPH, Natalie Schellack, PhD, and Michael P. Stevens, MD, MPH
Chapter Editor: Gonzalo Bearman, MD, MPH
Print PDF
KEY ISSUES
The inappropriate use of antimicrobials in human medicine is widespread. This has a direct impact on antimicrobial resistance, one of the greatest threats to global health, food security, and development today.1 The primary goal of antimicrobial stewardship, as defined by the Infectious Diseases Society of America (IDSA), is to optimize clinical outcomes while minimizing unintended consequences of antimicrobial use, including toxicity, the selection of pathogenic organisms (such as Clostridioides difficile), and the emergence of resistance.2
KNOWN FACTS
- Inappropriate use of antimicrobials is a global health problem of monumental proportions. In the United States, 30-50% of antimicrobials prescribed are unnecessary or inappropriate. Similarly, in countries where antimicrobials can be purchased without a prescription and antimicrobial use lacks regulation, antimicrobials are often over-prescribed by health workers and over-used by the public.1,3
- Indiscriminate use of antimicrobials is a major factor in promoting antimicrobial resistance.4
- The antimicrobial armamentarium is rapidly diminishing and must be preserved through judicious use, strict regulation, and supplementation with new agents to combat multi-drug resistant bacteria.5,6
- The combination of antimicrobial stewardship programs (ASPs) and infection control programs have been shown to curtail the emergence and transmission of antibiotic resistance.2
- Reducing inappropriate antimicrobial use has been shown to decrease healthcare costs, Clostridioides difficile infections, and improve patient outcomes.2,7
CONTROVERSIAL ISSUES
• Antimicrobial cycling, which involves the deliberate withdrawal of an antimicrobial or antimicrobial class from general use coupled with the substitution of antimicrobials from a different class with similar activity, is not a recommended stewardship strategy to curtail antimicrobial resistance. Studies have failed to provide sufficient evidence of benefit, and have proved that cycling is labor intensive, challenging, and an impractical use of resources.4
• Passive education (pamphlets, posters, etc…) alone is insufficient when used in isolation and must be coupled with other stewardship activities (see below) to be successful.4
• Low- to middle-income countries (LMICs) are likely to have limited staffing available to form an ASP. Lack of personnel has been identified as a common barrier to ASP creation in LMICs.8
• Shortages of antimicrobials and/or cost of medications may pose a significant barrier to antimicrobial stewardship in LMIC. In many LMICs broad-spectrum agents may be more readily available than more narrow-spectrum antimicrobials.9 LMIC must have a national focus in ensuring availability of antimicrobials for optimizing antimicrobial stewardship.
• Widespread availability of over the counter antimicrobials may pose a barrier to antimicrobial stewardship in LMICs. Public education campaigns have shown some effectiveness in limiting inappropriate use of over the counter antimicrobials in these settings.5
SUGGESTED PRACTICE
Hospitals are encouraged to implement a multidisciplinary antimicrobial stewardship team that includes among its core members, when available, an infectious diseases physician and clinical pharmacist with infectious diseases training. Other important members of this team would optimally include a hospital epidemiologist, a clinical microbiologist, an information system specialist, and an infection control professional.7 The United States Centers for Disease Control and Prevention suggest the following seven core elements be incorporated into all ASPs.7
- Leadership Commitment: Dedicating necessary human, financial and information technology resources.
- Accountability: Appointing a single leader responsible for program outcomes. Experience with successful programs show that a physician leader is effective.
- Drug Expertise: Appointing a single pharmacist leader responsible for working to improve antimicrobial use.
- Action: Implementing at least one recommended action, such as antimicrobial prospective audit and feedback of antimicrobial preauthorization.
- Tracking: Monitoring antimicrobial prescribing and resistance patterns.
- Reporting: Regular reporting of information on antimicrobial use and resistance to doctors, nurses and relevant staff.
- Education: Educating clinicians about resistance and optimal prescribing.
The following strategies have been proven to be effective ASP initiatives and should be considered as local resources allow:
- Pre-prescription Authorization (PPA)2,4,7
- Restrict the use of certain antimicrobials based on known overuse/misuse, spectrum of activity, toxicities, and cost.
- Post-prescription review with feedback (PPRF)2,4,7
- Conduct external reviews of antimicrobial therapy currently being administered in the hospital.
- Reviews and feedback should be performed by an expert in the field of antibiotic use
- Develop policies to optimize antimicrobial use.2,4,7
- Document dose, duration, and indication for all courses of antimicrobials.
- Develop hospital specific treatment guidelines for common scenarios (ie; surgical prophylaxis, diarrhea, pneumonia, urinary tract infection) that correspond to national standards, local drug formulary, and local resistance patterns.
- Antibiotic “Time outs” 2,4,7
- When microbiological results are available, typically within 24-48 hours, antimicrobials should be reassessed for opportunities to de-escalate, discontinue, or tailor therapy in light of new information.
- A re-assessment for the continuing need for ongoing antimicrobial therapy (and/or opportunities to de-escalate therapy to more narrow-spectrum antimicrobials) should occur at least every 48 hours while antimicrobials are continued.
- Pharmacy-driven Interventions2,4,7
- Intravenous to oral conversion of antibiotics known to have high oral bioavailability such as trimethoprim-sulfamethoxazole, fluconazole, and fluoroquinolones.
- Antimicrobial dose adjustments for renal and hepatic dysfunction.
- Pharmacokinetic and dynamic adjustments for selected medicines. Antimicrobial dose optimization to maximize penetration to organs and body tissues, activity against multidrug-resistant organisms (MDROs), and other pharmacokinetic interventions such as extended-infusion administration of beta-lactams where available.
- Alerts for common situations where antimicrobial therapy may be inappropriate or unnecessary.
- Examples: double anaerobic coverage, “bug-drug” mismatch, drug-drug interactions, prolonged empiric coverage without positive cultures, etc…
SUGGESTED PRACTICE IN UNDER-RESOURCED SETTINGS
Barriers to the effectiveness of stewardship in under-resourced areas (including LMIC) are many and include a paucity of human and technological resources, inadequate microbiology laboratory infrastructure and support, and insufficient funding, to name a few.10 Compounding the problem, the burden of infectious diseases and MDROs is higher and often times antimicrobials can be obtained without prescription, which makes the practice of antimicrobial stewardship more challenging.
- Efforts should be made to ensure that prescription from a trained professional is required whenever antimicrobials are administered.
- In areas where antimicrobial resistance testing is not (or is not widely) available, investing in lab capacity should be a priority at the national level.
- PPA is a strategy that may be less resource intensive than PPRF and has the potential for a significant impact on antimicrobial use.
- Access to antimicrobials classified as reserve under the WHO Aware classification should be restricted and their use ideally would be directed by susceptibility testing (Figure 1).11
- Developing antimicrobial prescription guidelines and PPRF pertaining to surgical antimicrobial prophylaxis are low cost and proven effective ASP interventions in LMICs.11,12
- Antibimicrobial therapy should always have an end date identified by condition.
- ASPs and infection prevention programs should be integrated (wherever feasible).
- Staffing models utilizing non-clinical pharmacists and/or nurses can be utilized in these settings as needed.13
- Antimicrobial resistance does not respect borders and is often difficult to detect. A collaborative, international approach to curbing resistance and expanding access to ASPs is of critical importance.
- Resources are available for establishing ASPs in LMIC such as the WHO 2019 toolkit on ASP in LMIC and the ISID position paper on global ASP with a focus on LMICs.11,14
- International programs such as the Global Antimicrobial Resistance Partnership (GARP) (https://cddep.org/wpcontent/uploads/2017/06/garp_factsheet-1.pdf) and the Global Antimicrobial Resistance Surveillance System (GLASS) (https://www.who.int/glass/en/) are available to assist with antimicrobial resistance surveillance and reporting.15
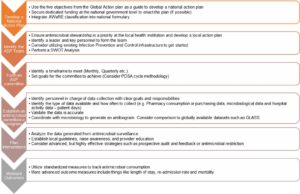
- Critical steps for creating an ASP are outlined in Figure 2.
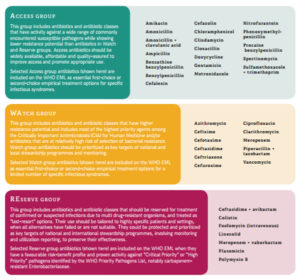
- Figure 1: World Health Organization AWaRE classification of antimicrobials. Figure from World Health Organization Antimicrobial Stewardship Programmes in Health-Care facilities in low- and middle-income countries – A WHO Practical toolkit.11
- Figure 2: Implementing an antimicrobial stewardship program.14
ANTIMICROBIAL STEWARDSHIP AND COVID-19
As of August 23rd 2020 there were over 23 million cases and nearly 806,000 deaths from the virus known as SARS-CoV2, the source of the COVID-19 pandemic.16 Infection prevention programs have a historical role in outbreak response, whereas this is not the case with ASPs. ASPs have the potential to impact COVID-19 response efforts in myriad ways, including in the creation of treatment guidelines, assessing for antimicrobial overuse, monitoring and managing drug shortages, et cetera17(Figure 3). It has been postulated that COVID-19 could lead to increased antimicrobial prescribing and thus increased resistance, although the full effects of this remain to be seen.18 Additionally, resources required to face the COVID-19 pandemic could delay expansion of ASPs, which is likely to be most significant in low- to middle-income countries. Antimicrobial stewardship in LMIC could also include drug formulary management to ensure that there is enough stock available to treat other infectious diseases (e.g. HIV, tuberculosis, malaria, et cetera).
Figure 3: Potential antimicrobial stewardship activities focused on COVID-19 (not all inclusive). *For medications utilized in the treatment of COVID-19.
SUMMARY
Antimicrobial resistance is an escalating global threat projected to cause more deaths than cancer by 2050. Antimicrobial stewardship is a key strategy to combat antimicrobial resistance, optimize clinical outcomes, and minimize the collateral damage of antimicrobial use. Pre-prescription authorization (restriction) of antimicrobials and post-prescription review with feedback (PPRF) are the most effective strategies. Hospitals should deploy multidisciplinary antimicrobial stewardship teams with an infectious diseases physician and clinical pharmacist with infectious diseases training wherever resources allow. Excellent resources exist to assist in creating ASPs, including in resource-limited settings.
REFERENCES
- Antibiotic Resistance: Fact Sheet. World Health Organization. https://www.who.int/en/news-room/fact-sheets/detail/antibiotic-resistance. Published 2018.
- Dellit TH, Owens RC, Mcgowan JE, et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America Guidelines for Developing an Institutional Program to Enhance Antimicrobial Stewardship. 2007;44:159-177.
- Fridkin S, Baggs J FR. Vital signs: improving antibiotic use among hospitalized patients. Morb Mortal Wkly Rep. 2014;63(9).
- Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an Antibiotic Stewardship Program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. 2016;62:51-77. doi:10.1093/cid/ciw118
- O’Neill J. Tackling drug-resistant infections globally: Final report and recommendations. Rev Antimicrob Resist. 2016:84.
- Policy Statement on Antimicrobial Stewardship by the Society for Healthcare Epidemiology of America (SHEA), the Infectious Diseases Society of America (IDSA), and the Pediatric Infectious Diseases Society (PIDS). Infect Control Hosp Epidemiol. 2012;33(4):322-327. doi:10.1086/665010
- CDC. The Core Elements of Hospital Antibiotic Stewardship Programs: 2019. Atlanta, GA: US Department of Health and Human Services, CDC. http://www.cdc.gov/getsmart/healthcare/implementation/core-elements.html. Published 2019.
- Howard P, Pulcini C, Levy Hara G, et al. An international cross-sectional survey of antimicrobial stewardship programmes in hospitals. J Antimicrob Chemother. 2014;70(4):1245-1255. doi:10.1093/jac/dku497
- Frost I, Craig J, Joshi J, Faure K, Laxminarayan R. Access Barriers to Antibiotics. Washington, DC Cent Dis Dyn Econ Policy. 2019:26.
- Aryee A, Price N. Antimicrobial stewardship – can we afford to do without it? Br J Clin Pharmacol. 2015;79(2):173-181. doi:10.1111/bcp.12417
- Geneva: World Health Organization. Antimicrobial Stewardship Programmes in Health-Care Facilities in Low- and Middle-Income Countries: A WHO Practical Toolkit. World Health Organization; 2019. licence: CC BY-NC-SA 3.0 IGO.
- Bardossy AC, Zervos J, Zervos M. Preventing Hospital-acquired Infections in Low-income and Middle-income Countries: Impact, Gaps, and Opportunities. Infect Dis Clin North Am. 2016;30(3):805-818. doi:10.1016/j.idc.2016.04.006
- Brink A, Van den Bergh D, Mendelson M, Richards GA. Passing the baton to pharmacists and nurses: New models of antibiotic stewardship for South Africa? South African Med J. 2016;106(10):947. doi:10.7196/SAMJ.2016.v106i10.11448
- Pierce J, Apisarnthanarak A, Schellack N, et al. Global Antimicrobial Stewardship with a Focus on Low- and Middle-Income Countries. Int J Infect Dis. 2020;96:621-629. doi:10.1016/j.ijid.2020.05.126
- World Health Organization. Global Antimicrobial Resistance Surveillance System (GLASS) Report. Geneva: World Health Organization.; 2018. license: CC BY-NC-SA 3.0 IGO.
- The Center for Systems Science and Engineering (CSSE) at JHU. COVID-19 Global Cases. https://coronavirus.jhu.edu/map.html. Published 2020. Accessed August 23rd, 2020.
- Stevens MP, Patel PK, Nori P. Involving antimicrobial stewardship programs in COVID-19 response efforts: All hands on deck. Infect Control Hosp Epidemiol. 2020;41(6):744-745. doi:10.1017/ice.2020.69
- Hsu J. How covid-19 is accelerating the threat of antimicrobial resistance. BMJ2020;1983(May): doi:10.1136/bmj.m1983
-