A New Chapter in a Long Evolutionary Story
Since mid-2025, a newly emerging variant of influenza A(H3N2), referred to as subclade K (J.2.4.1), has drawn global attention. Genetic and antigenic analyses show that this variant differs substantially from the H3N2 strain included in current seasonal influenza vaccines, with approximately ten amino-acid substitutions in the hemagglutinin (HA) protein, several affecting key antigenic sites [1]. These changes raise concerns about immune escape and potential impacts on vaccine effectiveness.
To understand why subclade K matters, it is helpful to place it within the broader evolutionary history of H3N2.
The Emergence of H3N2 and the 1968 Hong Kong Flu
Influenza A viruses are classified according to their surface glycoproteins, hemagglutinin (HA) and neuraminidase (NA). The H3N2 subtype emerged in 1968 following an antigenic shift, when a reassortment event introduced a novel H3 gene of avian origin into a human-adapted virus. This event led to the Hong Kong flu pandemic, which caused an estimated one million deaths worldwide [2].
Unlike some pandemic influenza viruses that disappear after initial waves, H3N2 successfully adapted to sustained human transmission and became established as a seasonal influenza virus.
Antigenic Drift and Accelerated Evolution
Following its emergence, H3N2 evolution has been driven largely by antigenic drift, characterized by the gradual accumulation of mutations that allow the virus to evade population immunity [3]. By the 1990s, increasing immune pressure from repeated infections and vaccination accelerated this process, leading to the diversification of H3N2 into multiple genetically distinct lineages [4].
Compared with other seasonal influenza subtypes, H3N2 has demonstrated a particularly high mutation rate, contributing to its well-documented ability to escape immunity and complicate vaccine strain selection.
The Fujian Flu and Lessons for Vaccine Mismatch
A pivotal moment in modern H3N2 history occurred during the 2003–2004 influenza season, when the A/Fujian/411/2002-like virus caused widespread outbreaks despite vaccination [5]. Antigenic mismatch between the circulating virus and the vaccine strain resulted in reduced vaccine effectiveness, highlighting the challenge posed by the rapid evolution of H3N2.
This episode reinforced the understanding that H3N2 often evolves faster than vaccine strains can be selected and produced, a challenge that persists today.
Post-COVID Re-emergence and the Rise of Subclade K
Global circulation of influenza viruses declined sharply during 2020–2022 due to COVID-19 control measures. While this temporarily suppressed transmission, it also led to reduced population immunity. As influenza activity rebounded in 2023–2024, H3N2 re-emerged into a more susceptible population, creating favorable conditions for rapid antigenic drift.
Against this backdrop, H3N2 subclade K has expanded across multiple regions. Early and intense influenza activity has been reported in parts of Asia, Europe, and North America [6]. Surveillance data from the United States indicate that H3N2 viruses are increasingly dominant, with a growing proportion belonging to subclade K.
In the Southern Hemisphere, increases in severe acute respiratory infections have prompted regional alerts and preparedness efforts [7], underscoring the broader context in which subclade K has emerged.
Early Signals from Pakistan: A Three-Season Comparison
Recent influenza surveillance from Pakistan highlights clear differences in seasonal timing and subtype dominance over the past three years. The 2023–24 season was marked by a short, intense epidemic driven predominantly by A(H3N2), with a sharp peak in late December and rapid decline thereafter. In contrast, the 2024–25 season showed delayed onset, with sustained activity emerging only in early to mid-December and dominated by A(H1N1)pdm09 and B/Victoria, resulting in a more prolonged epidemic profile.
The current 2025–26 season is following a distinct pattern, with early activity beginning in mid to late November and exclusive detection of A(H3N2) to date. Genomic data indicate substantial diversity within circulating H3N2 viruses, with subclade K (J.2.4.1) accounting for approximately half of sequenced cases. Together, these observations suggest an earlier H3N2-led season in 2025–26, shaped by ongoing antigenic drift and altered population immunity.
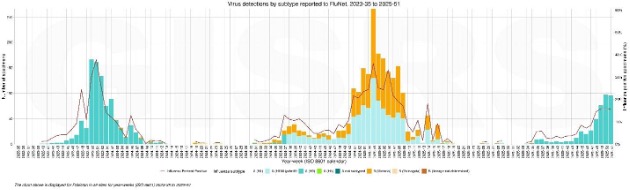
What Can We Expect in the 2025–26 Season in Pakistan?
The early dominance of A(H3N2), particularly subclade K, suggests that the 2025–26 season may continue to intensify as transmission progresses. Current positivity levels likely reflect early epidemic growth rather than peak activity, especially if subclade K continues to expand. Given the historical association of H3N2 with higher disease burden and rapid antigenic evolution, sustained transmission and increased clinical impact remain plausible. These trends reinforce the importance of continued epidemiological and genomic surveillance to inform risk assessment, vaccine performance evaluation, and preparedness efforts as the season unfolds.
What Subclade K Means for Vaccines
The emergence of subclade K has raised understandable concerns about vaccine effectiveness. Antigenic drift affecting HA antibody-binding sites has historically reduced vaccine performance against H3N2. However, early data from Europe are reassuring.
Preliminary estimates from the ECDC-coordinated Vaccine Effectiveness, Burden and Impact Studies (VEBIS) suggest moderate vaccine effectiveness, approximately 52–57% against laboratory-confirmed H3N2 infection during the early 2025–26 season despite subclade K dominance [8]. Similar findings have been reported from the United Kingdom and other European settings.
These data reinforce an important public health message: vaccine mismatch does not mean vaccine failure. Even when protection against infection is reduced, influenza vaccines continue to provide substantial protection against severe disease, hospitalization, and death, particularly among older adults and other high-risk groups.
Looking Ahead
The global emergence of H3N2 subclade K illustrates the virus’s remarkable evolutionary capacity and the ongoing challenges it poses for influenza control. Continued genomic and antigenic surveillance, timely vaccine updates, and sustained vaccination efforts remain essential to mitigate the impact of H3N2 in the current and future influenza seasons.
Written by ISID Emerging Leader, Dr. Massab Umair
Principal Scientific Officer and Head of Virology Department, National Institute of Health (NIH), Pakistan
